
While they’re in the public consciousness as weight loss jabs, semaglutide injections from the likes of Ozempic, Wegovy and Mounjaro were originally devised as type 2 diabetes medications.
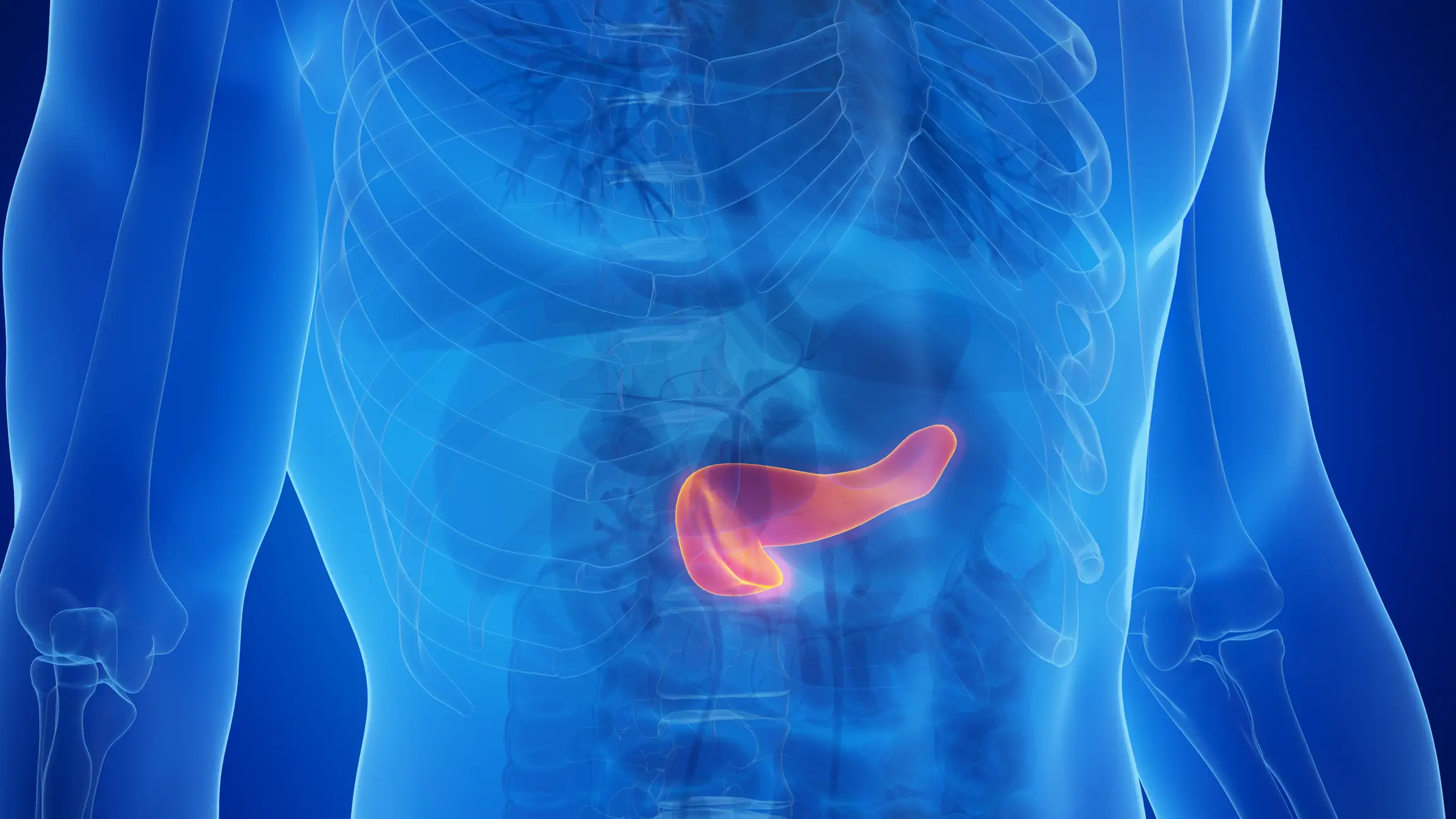
Type 2 diabetes is the result of your body becoming resistant to its own insulin. The pancreas produces insulin, a hormone that regulates blood sugar levels, and when you eat a lot of sugar it releases more insulin to help manage it within your blood.
Over time, heightened insulin release means your insulin receptors essentially get burned out and stop responding to the hormone. Without it, your body can’t regulate its blood sugar levels properly, and this can cause all kinds of nasty complications. This is type 2 diabetes, or the kind of diabetes that you can develop as a result of your lifestyle.

Advert
Left untreated, diabetes can lead to blood vessel damage, nerve damage, heart disease, stroeks, kidney disease, eye problems that can progress into blindness, and foot problems that can result in the need for amputation. The nerve damage can also trigger pain, numbness, tingling and digestive problems.
Semaglutide drugs were designed initially as a means to help manage blood sugar levels in people with type 2 diabetes. They work as appetite suppressors, meaning they can help to manage an individual’s hunger pangs and cravings, thereby serving as a platform for a well-controlled diet that minimises the likelihood of hypo- or hyperglycaemia.
Of course, those appetite-suppressing qualities are also great for losing weight. Ultimately, weight loss is all about calorie intake. Exercise is great and there are tonnes of reasons why you should do it, but in and of itself it’s not much use for weight loss.
Instead, weight loss is triggered by maintaining a calorie deficit. There are 3,500 calories in a pound of fat, so to lose that weight each week you would need to run a calorie deficit of 500 per day. That means burning 500 calories more than you consume. For the average person with a basal metabolic rate of 2,000 calories, that would mean limiting caloric intake to 1,500 calories per day.
It’s easy to say and hard to accomplish, requiring lifestyle changes that can be tough for some people to adapt to. With an appetite-suppressing drug, however, it’s easier to avoid snacking or breaking the rules of your diet either through cravings or compulsions, and so it’s easy to maintain that calorie deficit.
While they’ve taken the world by storm, it seems there may be some drawbacks to using these drugs as a weight loss solution rather than a diabetes management aid.

Hundreds of people have reported problems with their pancreas linked to taking weight loss and diabetes jabs, prompting health officials to launch a new study into side effects.
Some cases of pancreatitis reported to be linked to GLP-1 medicines (glucagon-like peptide-1 receptor agonists) have been fatal.
Data from the medicines regulator, the Medicines and Healthcare products Regulatory Agency (MHRA), shows that since the drugs were licensed there have been hundreds of cases of acute and chronic pancreatitis among people taking GLP-1 medicines.
This includes:
- 181 reported cases of acute and chronic pancreatitis linked to tirzepatide – the active ingredient for Mounjaro. Five people died.
- 116 reported reactions of this kind linked to liraglutide, one of which was fatal.
- 113 cases of acute and chronic pancreatitis linked to semaglutide – the active ingredient for Ozempic and Wegovy. One person died.
- 101 reported reactions of this kind linked to exenatide, three people died.
- 52 reported reactions of this sort linked to dulaglutide and 11 reported reactions lixisenatide. No fatalities were linked to either drug.
These cases are not confirmed as being caused by the medicines, but the person who reported them suspected they may be.
Nonetheless, Yellow Card Biobank project, launched by the MHRA and Genomics England, will see researchers examine whether cases of pancreatitis linked to GLP-1 drugs may be influenced by peoples genetic makeup.
The MHRA is calling for people who are taking GLP-1 medicine who have been admitted to hospital due to acute pancreatitis to submit a report to its Yellow Card scheme.
When a Yellow Card report is received, the MHRA will contact patients to ask if they would be willing to take part in the study.
Patients will be asked to submit more information and a saliva sample which will be assessed to explore whether some people are at a higher risk of acute pancreatitis when taking these medicines due to their genes.
GLP-1 agonists can lower blood sugar levels in people living with type 2 diabetes and can also be prescribed to support some people with weight loss.
Recent estimates suggest that about 1.5 million people in the UK are taking weight loss jabs.
Health officials have suggested that they can help to turn the tide on obesity, but have stressed they are not a silver bullet and do come with side effects.
Most side effects linked to the jabs are gastrointestinal including nausea, constipation and diarrhoea.
And the medical regulator recently warned that Mounjaro may make the oral contraceptive pill less effective in some patients.
Dr Alison Cave, MHRA’s chief safety officer, said: “Evidence shows that almost a third of side effects to medicines could be prevented with the introduction of genetic testing, it is predicted that adverse drug reactions could cost the NHS more than £2.2 billion a year in hospital stays alone.
“Information from the Yellow Card Biobank will help us to better predict those most at risk of adverse reactions – enabling patients across the UK to receive the safest medicine for them, based on their genetic makeup.
“To help us help you, we’re asking anyone who has been hospitalised with acute pancreatitis while taking a GLP-1 medicine to report this to us via our Yellow Card scheme.
“Even if you don’t meet the criteria for this phase of the Biobank study, information about your reaction to a medication is always extremely valuable in helping to improve patient safety.”
Professor Matt Brown, chief scientific officer of Genomics England, said: “GLP-1 medicines like Ozempic and Wegovy have been making headlines, but like all medicines there can be a risk of serious side effects.
“We believe there is real potential to minimise these with many adverse reactions having a genetic cause.
“This next step in our partnership with the MHRA will generate data and evidence for safer and more effective treatment through more personalised approaches to prescription, supporting a shift towards an increasingly prevention-focused healthcare system.”
FOODBible has approached Novo Nordisk for comment.
